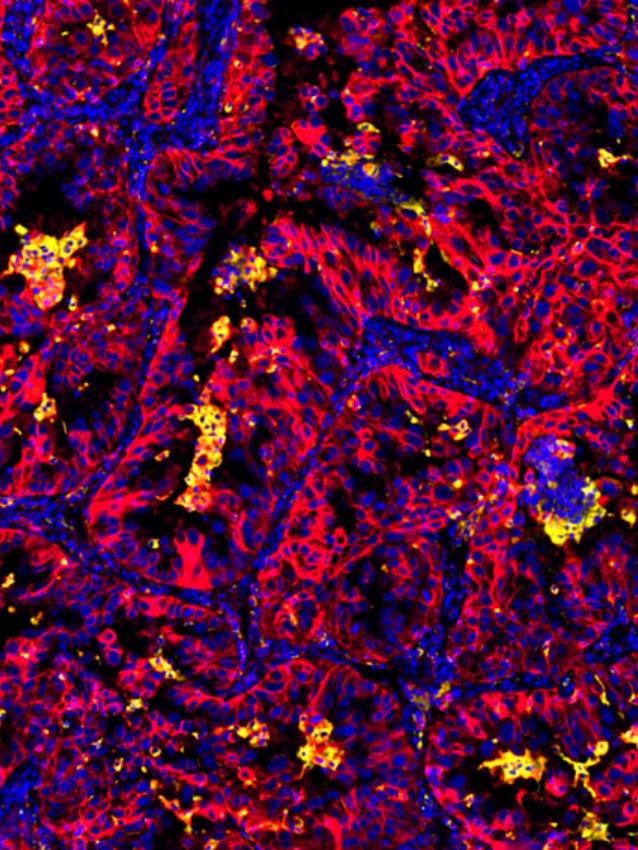
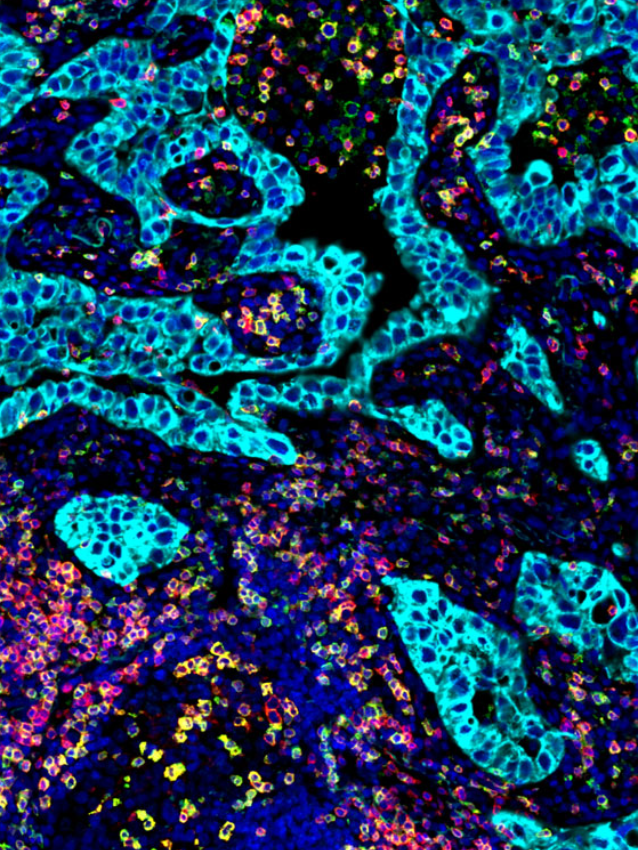
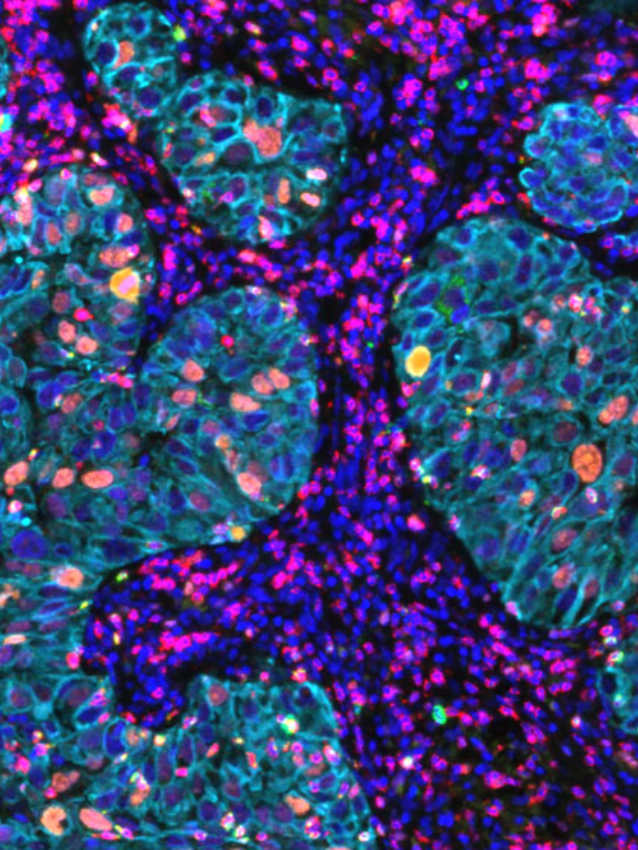
A Brief History of TiME
The brilliant Stephen Hawking once said that “The universe does not behave according to our pre-conceived ideas. It continues to surprise us.” The same statement is also true for how we think of the dynamic nature of the cancer universe, in particular the complexity of the tumor immune microenvironment (TiME/TMEs)- an ever-evolving rich milieu comprising of non-malignant immune cells, fibroblasts, adipocytes, extracellular matrix components and neuronal cell types.
October 27, 2021
Stephen Paget considered the founding father of TiME laid the foundations of this research area by formulating the seed and soil theory postulating that metastasis of a particular type of cancer (“the seed”) often metastasizes to certain sites (“the soil”) based on the similarity of the original and secondary tumor sites. Numerous studies in the 1970’s and 1980’s elaborated on the immune and angiogenic factors involved in this interplay of the tumor and its environment by characterizing the functions of cellular and humoral immune components. These studies established that these immune cells including T cells, B cells, NK cells and macrophages had the capacity to infiltrate solid tumors in humans and in animals (reviewed in Witz, 2009). Other early studies from Judah Folkman’s lab appreciated that tumor proliferation was dependent upon blood supply and that the interactions of tumor and endothelial cells initiated and drove this process. Angiogenic factors were then identified in various types of tumors and the possibility was raised that inhibiting such factors or their interaction with endothelial cells could perhaps be of clinical benefit to cancer patients (reviewed by Folkman J, 1972). Subsequent work by others also highlighted the interactions between the stroma and tumor cells. The stroma consists of the extracellular matrix (ECM), which is composed of proteoglycans, hyaluronic acid, and fibrous proteins such as collagen, fibronectin, and laminin; growth factors, chemokines, cytokines, antibodies, and metabolites; and mesenchymal supporting cells (e.g., fibroblasts and adipocytes), cells of the vascular system, and additional cells of the immune system. As tumors develop, the stroma also evolves.
It was a seminal paper 30 years ago that really changed the landscape of how we view the role of immune cells in the tumor microenvironment. van der Bruggen and colleagues first reported the existence of a human tumor antigen recognized by T-cells. They were able to clone the melanoma antigen-encoding gene (MAGE), which encodes an antigen recognized by cytotoxic T-cells, providing not only evidence that the immune system was capable of seeking and destroying tumor cells but also provided the first identification of a molecular target, one that is actively used clinically for advanced melanoma.
Today, the complexity and functional contribution of the tumor microenvironment in cancer progression much like our knowledge of the universe continues to expand at the cellular, organ and systemic level. This complexity is observed within not just the variety of cells involved, but also includes metabolism as a form of communication, aging and obesity as microenvironmental factors at the tissue and systemic levels, and the significance of gut microbiota. It is now appreciated that the composition of the tumor microenvironment plays a significant role not just in disease progression, but also in response to therapy, invasion, and metastasis or conversely, restraint of growth for many tumor types.
Recent work by Grunwald et. al., 2021 have discovered additional complexity to this vast cancer cosmos, with the identification of ‘‘subTMEs,’’ histologically definable tissue states anchored in fibroblast plasticity, with regional relationships to tumor immunity, subtypes, differentiation, and treatment response. They noted that “reactive’’ subTMEs, functionally coordinated fibroblast communities were immune hot and inhabited by aggressive tumor cell phenotypes yet appeared more chemo-sensitive. The matrix-rich ‘‘deserted’’ subTMEs instead harbored fewer activated fibroblasts and tumor-suppressive features but were markedly chemoprotective and enriched upon chemotherapy. Its clear from this study and others that we are still only just beginning to explore these new dimensions addressing the tumor-stroma-immune interplay in tumor progression, patient-specific stromal heterogeneity and how this is linked to clinical outcomes.
Want to explore this universe in your tissues? Check out some of our newest panels enabling a detailed interrogation of the tumor microenvironment associated with specific cancer types.
Learn more about our Panels
Characterize immune biology using pre-optimized panels and markers for whole slide analysis. Get unprecedented signal to noise, same slide H&E staining, fast turnaround time, and access to our team of assay development specialists to guide you. With every solution we offer, Ultivue is setting the new standard for mIF solutions.
See more